f orbital how many electrons|How many electrons can an orbital of type f hold? : Baguio Magnetic Spin, Magnetism, and Magnetic Field Lines. An atom with unpaired . Buy McAfee Total Protection, 5 Devices, 1 User, 1 Year Subscription Online at the best price and get it delivered across UAE. Find best deals and offers for UAE on LuLu Hypermarket UAEThe most authoritative resource for predictive analysis, expert picks, stats, and odds in the world! Our staff of expert handicappers work to help give you that competitive edge at the window.
PH0 · s,p,d,f Orbitals
PH1 · s, p, d, f Atomic Orbitals
PH2 · The periodic table, electron shells, and orbitals
PH3 · Shells, subshells, and orbitals (video)
PH4 · Orbitals Chemistry (Shapes of Atomic Orbitals)
PH5 · How many electrons can an orbital of type f hold?
PH6 · For s, p, d, and f orbitals, how many electrons can each hold?
PH7 · For s, p, d, and f orbitals, how many electrons can each hold?
PH8 · Electronic Orbitals
PH9 · 8.3: Electron Configurations
PH10 · 2.4 Electron Configurations
The easiest way to transfer files between Mega and Google Drive is by using a dedicated third party app. Applications like MultCloud, RiceDrive, and Cloud HQ are designed specifically for syncing .
f orbital how many electrons*******However, the electron can exist in spin up (m s = +1/2) or with spin down (m s = -1/2) configurations. This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, .
Magnetic Spin, Magnetism, and Magnetic Field Lines. An atom with unpaired .
How many electrons can an orbital of type f hold?Magnetic Spin, Magnetism, and Magnetic Field Lines. An atom with unpaired .As shown by the graphs, electrons of the s orbital are found closer to the nucleus .The four different types of orbitals (s,p,d, and f) have different shapes, and one .
Key Questions. How many electrons can s,p,d,f hold? Answer: 2,6,10,14 respectively. Explanation: If ℓ is the angular quantum number of subshell then maximum electrons it can hold is 2(2ℓ +1) Sub . The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. Orbital Energies and Atomic Structure. The energy of atomic orbitals increases .
Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Electrons in the same subshell have the same energy, while electrons in different shells or subshells have different energies. Created by Sal Khan. .
How many electrons can an orbital of type f hold? A. 6. B. 10. C. 2. D. 14. E. 1. Since there can be [-ℓ, ℓ] orientations and since the orbital type f has ℓ = 3, we should have 7 .21,441. According to the quantum atomic model, an atom can have many possible numbers of orbitals. These orbitals can be categorized on the basis of their size, shape or orientation. A smaller sized orbital means .
f orbital how many electrons How many electrons can an orbital of type f hold?Electronic Structure of Atoms. s, p, d, f Atomic Orbitals. In today’s post, we will talk about the atomic orbitals. So, first, what is an orbital? In a formal, quantum mechanical definition, orbitals are essentially probability .Figure 9.6.5 9.6. 5: Electrons are added to atomic orbitals in order from low energy (bottom of the graph) to high (top of the graph) according to the Aufbau principle. Principle energy levels are color coded, while sublevels are grouped together and each circle represents an orbital capable of holding two electrons.1 Answer. BRIAN M. May 26, 2014. The f orbital has 7 sub levels with the possibility of two electrons in each suborbital. Therefore, the f orbital can hold 14 electrons. Such overlaps continue to occur frequently as we move up the chart. Figure 8.3.1 8.3. 1: Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). Electrons .

1 Answer. The f sublevel as a whole can hold up to 14 electrons due to the fact that it consists of 7 orbitals, but each one can only hold up to 2 electrons. 2 electrons ---> see below: The f sublevel as a whole can hold up to 14 electrons due to the fact that it consists of 7 orbitals, but each one can only hold up to 2 electrons.How many electrons are present in the f block? Flexi Says: There are 7 f orbitals, and each orbital can hold a maximum of 2 electrons, the f block can accommodate a total of 14 electrons. Therefore, there are 14 electrons present in the f block for each element's respective electron configuration. Discuss further with Flexi.
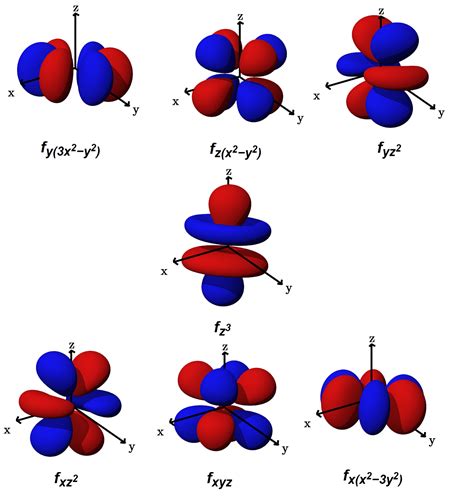
The maximum number of electrons an orbital can hold is two. So, the capacity of each subshell is: s-subshell : maximum of 2 electrons (as it contains only 1 orbital) p-subshell : maximum of 6 .7f atomic orbitals. For any atom, there are seven 7f orbitals. The f-orbitals are unusual in that there are two sets of orbitals in common use.The first set is known as the general set, this page.The second set is the cubic set, this page and these might be appropriate to use if the atom is in a cubic environment, for instance. Three of the orbitals are common to .
How many electrons can s,p,d,f hold? Chemistry Electron Configuration s,p,d,f Orbitals. 1 Answer Junaid Mirza May 9, 2018 #2, 6, 10, 14# respectively. Explanation: If . How many electrons can an f orbital have? How many electrons can there be in a p orbital? . These are arbitrarily given the symbols px, py and pz. This is simply for convenience; the x, y, and z directions change constantly as the atom tumbles in space. Figure 3: Hydrogen's electron - the 2p orbitals. The p orbitals at the second energy level are called 2p x, 2p y and 2p z. There are similar orbitals at subsequent levels: 3p x, 3p y .Fourteen would be the maximum number of electrons across an entire f-type sub-shell, but the question only asks about one orbital. answered. 11.9k. The question specifically ask that no.of electron an orbital of f subshell can hold.. As we know that f subshell contain 7 orbital and each orbital can hold maximum 2 electons so correct answer .
f orbital how many electrons The four chemically important types of atomic orbital correspond to values of ℓ = 0, 1, 2, and 3. Orbitals with ℓ = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. All orbitals with values of n > 1 and ell = 0 contain one or more nodes.
F orbitals are the orbitals that, in total, have the affinity to accommodate 14 electrons in them. The shape of the f orbital is tetrahedral. Though the shape of the f orbital is more complex than the other orbitals, the rule of filling the orbital remains the same as that of p and the d orbitals. The alignment of the electrons is also found to .The four chemically important types of atomic orbital correspond to values of l = 0, 1, 2, and 3. Orbitals with l = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring . The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 5.1.3 or 5.1.4 ). Thus, the electron configuration and orbital diagram of lithium are:10 d orbital electrons: 14 f orbital electrons: Visualizing Electron Orbitals. As discussed in the previous section, the magnetic quantum number (m l) can range from –l to +l. The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells. As shown in Table 1, the s subshell has one lobe, the p .The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 6.26 or Figure 6.27). Thus, the electron configuration and orbital diagram of lithium are: An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus.
The method of entering electrons into orbitals through the Aufbau principle is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. The first two electrons of fluorine enter the 1s orbital. The s-orbital can have a maximum of two electrons. Therefore, the next two electrons enter the 2s orbital. The p-orbital can have a maximum of six electrons.The diagram below shows the number of valence electrons (VE) for the main group elements. A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = .
Football at 365Scores. Football live scores by 365Scores, covering over 1,000 competitions with all today's matches of top competitions including UEFA Champions League, MLS and NWSL. You can also find detailed information about Real Madrid, FC Barcelona and Manchester City with latest results, fixtures, standings, news, highlights and .
f orbital how many electrons|How many electrons can an orbital of type f hold?